You are only 76% human [1].
This may feel strange to know, but you share your body with a rich and dynamic ecosystem of bacteria contained in your mouth, in your gastrointestinal tract, and on your skin [1].
These bacteria are not biologically inert: taken together, the trillions of microbes express 100-times as many genes as the human genome [2]. This relationship, termed the ‘extended genome,’ is the result of a co-evolution between our species and bacteria that is now recognised to be essential for human health [2][3].
In recent years, there has been an immense surge of interest in this complicated world of bacteria with whom we share our bodies. This complex field has led to some interesting hypotheses, learned insights, and – typical of nutrition and health science taken into the mainstream – hyperbole and some misinformation.
This article will take a look into the world of the microbiome, providing a definition for the terminology, a basic understanding of the structure of the ecosystem, and then a focus on the role of diet and nutrition in shaping and influencing the microbiome toward health or disease.
Defining Terms: What is the “Microbiome”?
First, let’s get the terminology down:
- The “microbiome”: the term for the ‘extended genome’ provided by the bacteria in the human gut, i.e. what functions do they perform?
- The “microbiota”: the term for the different bacteria in the ecosystem, i.e. what bacteria are there, and in what proportions?
- “Bacteria”: single-cell organisms that are highly adaptable.
- “Dysbiosis”: the term for disturbances in the composition of the microbiota, influencing disease states.
Where did this system come from, and why? At a basic level, we can say that as a species we provide a very attractive residence for different bacteria, from our UV-exposed, aerobic skin to the anaerobic, dark, moist, and energy-rich gut which is the primary residence for bacteria in humans [4].
In the course of their evolution, human beings have colonised every corner of the planet, adopting diverse diets in radically different natural environments and climates. Our gastrointestinal tract is one of the largest interfaces (250-400m2) with our external environment, providing for both the digestion and assimilation of essential nutrients, and the first line of immune defence for host health [5]. The exceptional diversity in our gut microbiota may thus reflect diversity of environment, food sources, and consequent adaptations influencing host health [4][5].
Whatever the evolutionary origins of this symbiotic relationship, bacteria like humans as a residence and – depending on how we shape them and feed them – can enhance our health or promote conditions which may precipitate disease.
To understand this more, let’s look at the structure of the system.
nbsp;
Structure, Composition & Function of the Human Microbiota
As an ecosystem of living organisms, the microbiota has taxonomic (the categorisation of living organisms) layers of organisation. At the broadest level, there are 4 main divisions, known as ‘phyla’: Firmicutes, Bacteroidetes, Actinobacteria and Proteobacteria [3].
These phyla are considered our “bacterial core,” with the majority of bacterial types belonging to two major phyla: the Firmicutes and Bacteroidetes, and significant contributions from Actinobacteria and Proteobacteria [6][7].
Within each phylum, there are a multitude of different genus, and within the genus, individual species. The following table contains two examples of this taxonomic organisation:
| Phylum: | Bacteroidetes | Firmicutes |
| Genus: | Prevotella | Lactobacillus |
| Species: | Prevotella copri (P.copri) | Lactobacillus acidophilus (L.acidophilus) |
While at a phylum level the human microbiota does not have as much variation as other ecosystems such as soil for example, it is within the composition of each phyla – at the genus and species level – that significant inter-individual variability is observed [3][4].
Diversification in the human gut thus reflects the depth and breadth of variability within each major phylum [3]. For example, in analysis of 124 Nordic and Mediterranean subjects, there was not one single abundant species shared between any 2 persons [6]. In each faecal sample, up to 160 bacterial species were identified in each sample, with a total of 3.3-million genes in the study cohort [6].
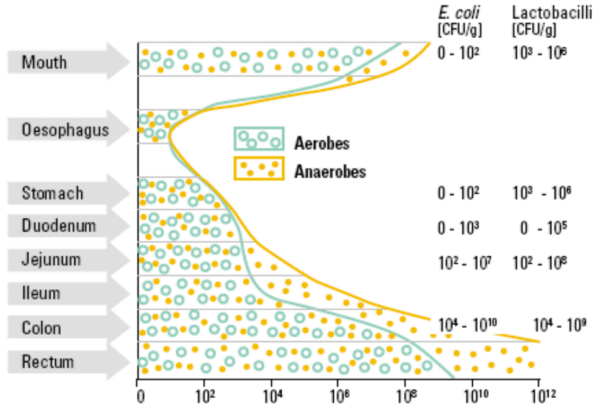
In relation to location, the primary site of bacterial colonisation in humans is in the large intestine. In the stomach and small intestine (duodenum, jejunum, and ileum), there are small numbers of primarily aerobic bacteria, but these compartments of the GI system are not major locations for bacteria [5]. The acidic environment of the stomach, pancreatic secretions and bile salts in the small intestine, coupled with the presence of oxygen and short transit time, create an environment that limits large-scale colonisation [8].
In the colon, however, there is increasing predominance of anaerobic bacterial species provided with fermentable substrates from the foods we consume, in particular complex carbohydrates which pass undigested through the small intestine. This coupled with a long transit time results in an extensively colonised large intestine with a dense and diverse bacterial community [8].
While our understanding of the full spectrum of functions of the microbiota is still emerging, a number of important roles have been elucidated which influence host health, including:
- Facilitating maturation of the epithelial layer of the intestines;
- Facilitating maturation of the immune system [intimately linked to a) above];
- Regulating inflammation;
- Synthesising of certain vitamins and minerals;
- Production of secondary metabolites which influence host health, e.g. short-chain fatty acid [SCFA] production in the colon [9][10][11][12][13].
- The variability of the microbiota between and within individuals reflects a dynamic system that is influenced by geographical variability, life-stage (including birth delivery and feeding method), age, antibiotic use, and diet [10]. These ongoing variables are associated with shaping, reshaping, and influencing the composition of the microbiota and as a consequence, health or disease processes [10].
Interacting with the gut-brain axis to influence neurological processes and mood;
Shaping the Microbiota
The composition of the microbiota is shaped from minute one of life. Whether the in-utero environment is sterile, and a foetus develops in a bacteria-free environment, is still a matter of debate [14].
The primary determinant of an infant’s bacterial community is birth delivery method. The microbiota of babies delivered via the birth canal reflects maternal vaginal composition, which is typically stable [14]. Conversely, the microbiota of babies delivered via caesarean-section reflects the bacterial composition of skin of the mother and delivery room attendees, and the microbiota of skin is highly variable [14].
There are marked differences in the composition of the microbiota, relative to delivery method. Birth canal deliveries are abundant in Lactobacillus and Prevotella genus, while C-section deliveries are abundant in Staphylococcus [15]. While the microbiota of a birth canal-delivered infant will reflect maternal composition within 3-7 day post-delivery, the microbiota of a C-section delivered infant may be disturbed for up to 6-months [15]. While the long-term consequences of such an altered microbiota are yet emerging, C-section deliveries are associated with higher prevalence of asthma and allergy, and certain autoimmune conditions, in particular Coeliac and Diabetes Type-1 [15].
Feeding method follows on from delivery method as a vital early life-stage variable influencing the microbiota. An important feature of the microbiota in this life-stage, contrasted with the adult microbiota, is that the composition of the infant microbiota is not intended for diversity [16]. The microbiota of breastfed infants is dominated by specific species of the Bifidobacterium genus, specialising in the degradation of human milk oligosaccharides [16].
Conversely, formula-fed infants display higher levels of pro-inflammatory Proteobacteria, resembling adult patterns of colonisation – which is not a positive feature for the infant gut that thrives on selective functions of a limited number of species [16]. In breastfed infants, these species – in particular Bifidobacteria – utilise the complex sugars found in human milk to populate the gut, express anti-inflammatory genes, increase host immune tolerance, and enhance gut barrier function [16].
Geographic region is another important variable; however, it is arguable that the primary difference here is the composition of traditional diets vs. Western diets. In comparative analysis of populations from Malawi, Peru, and Philadelphia, the US subjects exhibited the least microbial diversity [11]. While the African and South American subjects were distinguishable, the distinction was not as extreme as between the traditional diets vs. the Western diet: both African and South American groups were dominated by Prevotella, a genus of specialised fibre-degrading bacteria [11].
The analysis also confirmed that the composition of the adult microbiota evolves over the first 3-years of life, after which it is relatively stable [11]. In the infants in the cohort, however, Bifidobacterium dominated in all subjects, indicating that shifts to an adult composition are strongly influenced by feeding method and duration, and habitual diet following weaning [11].
These early life-stage variables are largely determined for us. However, the significant differences in the microbiota of African and South American subjects consuming traditional diets, and US subjects consuming a Western diet, indicates that diet is a primary driver of variation in the human microbiome [11][17].
The Influence of Diet on the Human Microbiome
Alterations in the composition of the microbiota reflect humans’ ability to respond to nutritional changes in the short and long-term. Microbes are specialised in the fermentation of different dietary substrates, thus dietary choices and patterns provide substrates for the selective growth of specific species [17].
In both African children and adults consuming a diet low in animal protein and fat, and high in fibre and non-starch polysaccharides [NSP], high levels of Prevotella and other microbial species required for metabolism of indigestible carbohydrates are found consistently [18][19]. The microbiome of populations consuming diets rich in indigestible carbohydrates display greater microbial diversity than Western diets, which corresponds to the diversity of complex carbohydrate structures, including resistant starch [RS], prebiotic fibres and other NSP, in traditional diets, and is associated with host health [20][17].
The benefit of diets high in fibre is broadly attributable to structurally diverse, non-digestible carbohydrates, including resistant starch [RS], NSP, and other prebiotic fibres which reach the colon and undergo selective microbial fermentation [21]. High intake of fibre/NSP results in a shift to increased populations of short-chain fatty acid [SCFA] producing microbes [22][18]. SCFA’s, in particular butyrate, exert anti-inflammatory effects in the colon, and are associated with inhibition of tumorigenesis, carcinogenic detoxification, and antineoplastic activity [19].
Conversely, ‘Western’ diets high in fat, sugar, animal protein, and low in fibre, negatively impact the microbiome, with effects seen as early as one year of age [18]. European children consuming a Western diet showed 2-times more abundant levels of Firmicutes and lower Bacteroidetes, and had significantly lower levels of SCFA [18]. This profile is associated with increased levels of pro-inflammatory proteobacteria, and increased secondary bile acid metabolites, which are potentially carcinogenic and increase levels of pathogenic bile-tolerant bacteria [23][24]. These changes are driven by specific characteristics of the Western diet. High fat intake alters the microbiome through driving increased bile acid production and increasing bile-acid tolerant bacteria levels (20). However, fat subtype is important. The evidence suggests monounsaturated fats do not significantly influence microbial composition [25][24]. Omega-3 fish oil polyunsaturates may increase some beneficial Actinobacteria and Lactobacilli, and modify bile acid composition [24][25].
High animal protein intake is another feature of the Western diet and is associated with increased protein putrefaction into potentially toxic and carcinogenic metabolic by-products, decreased SCFA’s, and decreases in butyrate-producing bacteria [20]. However, protein is also strongly correlated with increased microbial diversity [24], and beneficial compounds produced through proteolytic fermentation [7]. The detriment to high animal protein is mediated by the presence or absence of fibre, and has been shown where a high protein intake is coupled with fibre of 8-12g/d [26]. With fibre intake at 31g/d, no evidence of increased putrefaction, toxic metabolites, or decrease in SCFA’s was noted despite high protein intake [27]. Alterations in the microbiome are thus primarily associated with the presence or absence of fibre. [21]
Influence of the Microbiome on Intestinal Disease
In human subjects, feeding an exclusively animal-based, high saturated fat and no-fibre diet rapidly shifted subjects’ microbiota to increased secondary bile acid metabolism, which corresponded to inhibition of the Firmicutes and Bacteroidetes phyla, and increased B.wadsworthia [22]. Inhibition of the Firmicutes phyla corresponds to increased bile-tolerant and pro-inflammatory proteobacteria and nitrogenous metabolites, associated with intestinal inflammation and colon cancer. [23]
Subjects with Inflammatory Bowel Disease [IBD] display low microbial diversity, reduced levels of Firmicutes and Bacteroidetes, and low levels of microbe-derived butyrate [24]. These effects are mediated by low-fibre/NSP diets, which reduce SCFA-producing bacteria, compromise intestinal tight-junction integrity, and facilitate pathogenic adhesion to intestinal cells [23][28]. Adhesion to mucosal epithelial cells by pathogenic E.coli is implicated in the pathogenesis of IBD, in particular Crohn’s Disease [CD] and colon cancer [28][29]. Common dietary emulsifiers, ubiquitous in the Western diet, have been implicated in altering mucosal-bacterial interactions, facilitating pathogenic translocation across the mucosa and adherence to epithelial cells, stimulating pro-inflammatory immune responses. [30]
High-fat Western diets increase bile acid production which, in the absence of fibre, passes to the colon and undergoes metabolism to secondary bile acids associated with colon cancer [19][23]. In a diet-swap study, African-American subjects consuming a Western diet showed increased expression of microbial conversion of bile acids into pro-carcinogenic secondary metabolites, while age-matched native Africans consuming a high-fibre/NSP diet showed an abundance of SCFA-producing bacteria and high butyrate levels [19]. Switching African-American and native African subjects’ diets resulted in a reversal of these effects [31]. Cumulatively, this demonstrates a clear association between diet and colon cancer [19][31]. In addition, increases in taurine-conjugated bile acids from a high saturated fat intake mediates B.wadsworthia growth, which is strongly implicated in IBD. [25][28]
Influence of the Microbiome on Obesity
An observation of obesity research is that the composition of the microbiota differs between lean subjects and those who are obese, with obese subjects showing increased populations of Firmicutes and decreased populations of Bacteroidetes [32]. These effects reverse with weight loss and calorie restriction [33], indicating that alterations in the microbiota are driven by diet and increasing adiposity. [34]
The argument that has arisen is whether obesity is consequent on shifts in the microbiota, or whether the shift in the microbiota is a consequence of obesity. In this respect, the comparative study between European children and African children is instructive: the European children displayed twice the abundance of Firmicutes and lower Bacteroidetes, the composition observed in obese adults [18]. This supports the primacy of diet as the underlying driver of change. However, it also suggests that the change may precipitate metabolic dysregulation in obesity.
A feature of the microbiome is that it displays circadian rhythmicity: a high-fat and high-sugar Western diet dysregulates the rhythmic regulation of intestinal barrier function, and promotes metabolic dysfunction [34]. Alterations in the microbiota as a consequence of a Western diet pattern may increase energy harvesting from food, evident through increased activity of enzymes involved in energy extraction [7]. Alterations in bile acid metabolism may increase triglyceride synthesis and lipid storage [7]. A trial in humans using faecal transplant found that obese subjects receiving the transplant increased insulin sensitivity measured via insulinemic clamp [35]. However, it is important to state that the majority of the aforementioned proposed mechanisms are derived from animal models. [34]
While there is little doubt that the composition of the microbiota influences metabolic function, it is a distinct overreach to suggest – as some have – that obesity is caused by shifts in the microbiome. The evidence shows that the shift in composition is driven by diet, with the Western diet driving alterations in the microbiota as early as childhood [18]. Obesity is driven by the same diet pattern, as well as chronic caloric excess [36]. The two are not mutually exclusive. A human feeding study found that in subjects with Metabolic Syndrome, consumption of a Mediterranean diet pattern for two years elicited significant changes in the microbiota that corresponded to improved plasma glucose and triglyceride levels [37]. Taken as whole, a more accurate description of the role of the microbiota in obesity/metabolic disease is that the alteration in bacterial composition, driven by diet, exacerbates the deleterious effects of increasing adiposity by promoting inflammation, insulin resistance, and altered fat metabolism. [34][35][37]
The Influence of the Microbiome on Brain Health
The gut-brain-microbiota axis includes the central nervous system, the autonomic nervous system, and the enteric nervous system; bi-directional communication allows for the brain to influence functions in the gut, and for the gut to influence brain function [38]. Within this axis of communication, the microbiota has emerged as a factor influencing neurological processes. [38]
Bacteria in the gut modulate the activity of the enzyme indolamine oxidase [IDO], which is the rate-limiting step in metabolism of the dietary amino acid tryptophan, responsible for synthesis of the neurotransmitter serotonin [39]. In the presence of dysbiosis, high IDO activity diverts tryptophan metabolism to the kynurenine pathway and away from serotonin production [39]. This may be implicated in depression, and symptoms of Irritable Bowel Syndrome. [39][40]
In addition, the production of SCFA by colonic bacteria may have important extra-intestinal benefits, including neuroactive properties [41]. Emerging evidence suggests bacteria may influence Brain-Derived Neurotropic Factor [BDNF], a pathway regulating neurogenesis, learning and memory, and increasing BDNF expression through SCFA. [39]
Perhaps the most interesting evidence in humans to date is the ability of the microbiome to modulate stress. In humans, consuming a fermented milk strengthened with probiotic bacteria improved emotional processing, assessed using functional magnetic resonance imaging [fMRI] [42]. In another trial in healthy subjects, one month of probiotic supplementation reduced urinary cortisol output, suggesting modulation of stress responses [43]. A particular underlying mechanism of interest is the capacity of the microbiota to modulate neuroinflammation, an effect which may be mediated by SCFA [44]. Modulating gut bacteria has been shown to reduce anxiety and depression, effects attributed to reductions in neuroinflammation. [45]
There are suggestive associations between the microbiome and Autism Spectrum Disorder [ASD], as altered gut microbiota is observed in children with ASD, and gastrointestinal symptoms consistently correlate with ASD symptom severity [46]. Children with ASD have high levels of the pathogenic Clostridia strain, which may increase the activity of neurotoxin-producing bacteria [46]. The strong prevalence of GI disturbances in children with ASD suggests a gut-brain interaction [47]. However, to date, data on the role of the microbiome in ASD is limited: currently, a large-scale RCT is underway in Italy examining the effects of probiotic supplementation in children with ASD [48] – we await the findings.
Feeding Your Gut Bugs
The fundamental dietary constituent promoting a positive microbiota composition is dietary fibre [21]. Within the classification of fibre, certain prebiotic compounds are particularly beneficial. Gibson et al. [49] defined prebiotics as: “a selectively fermented ingredient that allows specific changes, both in the composition and/or activity of the gastrointestinal microbiota that confers benefits.” The key characteristics of prebiotics include resistance to breakdown by gastric acid, resistance to enzymatic degradation and absorption in the small intestine, and selective fermentation, resulting in growth and/or activity of the microbiota and associated health benefits [49].
Prebiotics naturally occur in food, notably garlic, onions, wheat, oats, soy, chicory, asparagus, Jerusalem artichokes and leeks [50]. Classification as prebiotic is to date confined to inulin, oligofructose [FOS] and galactooligosaccharides [GOS], for which there is evidence of benefits to human gastrointestinal health [51]. The primary target of prebiotic fermentation is the Bifidobacterium genera, which are present in greater amounts in the colon, and preferentially ferment prebiotic oligosaccharide fibres [52]. Increased production of SCFA is one mechanism through which prebiotics may improve intestinal health [53].
Non-starch polysaccharides are another important form of fibrous, non-digestible carbohydrate. In particular, pathogenic E.coli adherence to intestinal epithelial cells can be inhibited by NSP, and research has shown that NSP from green plantains and broccoli blocks E.coli adherence at physiologically relevant concentrations [30][54]. This suggests a preventative ‘contrabiotic’ effect against a mechanism implicated in the pathogenesis of CD and colon cancer (30; 54). Dietary NSP confer prebiotic effects, selectively feeding the growth of Bifidobacteria and Lactobacilli spp., an effect associated with increased SCFA production, and butyrate-mediated reductions in inflammation and carcinogenesis [23][53].
It is important to note that many of the observed protective benefits of dietary fibre are observed with a minimum threshold of 30g/d [21][27], and many of the traditional diets observed fall into 40-50g/d range [19][31]. This suggests that the optimal diet for gut health is one which includes a diverse array of quality carbohydrate sources.
Aside from dietary fibre, modifying the fat composition of the diet may be an important step – although the data here is limited. What we do know is that high saturated fat intake increases bile acid production and acid-tolerant bacteria [20][24]. A ‘Mediterranean’ fat profile, enriched in unsaturated fats, may inhibit pathogenic bacteria group by shifting composition of bile acid [24][25]. Modifying the ratio of omega-6 polyunsaturates to omega-3 polyunsaturates, with elevated omega-6 associated with inflammation, through specifically increasing omega-3’s, has shown to be effective in maintaining disease remission in IBD [55][56].
One final piece of the microbiome puzzle is the interaction between vitamin D and gut health. The Vitamin-D Receptor [VDR] is highly expressed in intestinal tissues, and there are direct interactions between the VDR and microbiota which enhance host immunity [57]. Mechanistically, there is emerging evidence that vitamin D enhances the capacity of immune cells to kill mucosally translocated pathogenic E.coli [58]. Through the VDR-microbiota interaction, vitamin D exerts immunological effects in the gut including inhibition of inflammatory cytokines, increased antimicrobial activity, maintenance of intestinal barrier integrity, and prevention of dysbiosis [57].
Conclusion
A high animal protein, high animal fat, low-fibre diet appears, on the totality of evidence in relation to the human microbiome, to be profoundly deleterious. Until evidence emerges to the contrary, I believe the burden of proof is on advocates of such diets to demonstrate the opposite. Structurally diverse, non-digestible carbohydrates – broadly known as ‘fibre’ – play a fundamental role in shaping the human microbiome to a composition that enhances host health across a range of body systems, from gut to brain.
In summation:
- Alterations in the human gut microbiome are driven primarily by the presence or absence of fibre;
- Regardless of your early life-stage variables, maintaining a high – 30g/d+ – fibre intake can help shift the composition of your gut bacteria toward beneficial, fibre-degrading, SFCA-producing, bacterial species;
- It is particularly important to reach this threshold of fibre intake if you consume a habitual high-protein diet;
- Feed specific ‘prebiotic’ foods – garlic, onions, wheat, oats, soy, chicory, asparagus, Jerusalem artichokes and leeks;
- Feed specific ‘contrabiotic’ foods – under ripe bananas, plantains, or broccoli;
- Focus dietary fat intake primarily on unsaturated fats.
Recall that the adult microbiota is relatively stable, i.e. resistant to change. What this shows is that in order to sustain a certain composition, the dietary intervention must be sustained.
References
- Sender, R., Fuchs, S. and Milo, R. (2016). Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLOS Biology, 14(8), p.e1002533.
- Lederberg, J. (2000). Infectious History. Science, Apr 14;288(5464), pp.287-93.
- Turnbaugh, P., Ridaura, V., Faith, J., Rey, F., Knight, R. and Gordon, J. (2009). The Effect of Diet on the Human Gut Microbiome: A Metagenomic Analysis in Humanized Gnotobiotic Mice. Science Translational Medicine, 1(6), pp.6ra14-6ra14.
- Ley, R., Knight, R. and Gordon, J. (2007). The human microbiome: eliminating the biomedical/environmental dichotomy in microbial ecology. Environmental Microbiology, 9(1), pp.3-4.
- Thursby, E. and Juge, N. (2017). Introduction to the human gut microbiota. Biochemical Journal, 474(11), pp.1823-1836.
- Qin, J., Li, R., Raes, J., Arumugam, M., Burgdorf, K. S., Manichanh, C., Wang, J. (2010). A human gut microbial gene catalog established by metagenomics sequencing. Nature, 464(7285), 59–65.
- Lopez-Legarrea, P., Fuller, N., Zulet, M., Martinez, J. and Caterson, I. (2014). The influence of Mediterranean, carbohydrate and high protein diets on gut microbiota composition in the treatment of obesity and associated inflammatory state. Asia Pac J Clin Nutr., 23(3), pp.360-8.
- Donaldson, G., Lee, S. and Mazmanian, S. (2015). Gut biogeography of the bacterial microbiota. Nature Reviews Microbiology, 14(1), pp.20-32.
- Kinross, J., Darzi, A. and Nicholson, J. (2011). Gut microbiome-host interactions in health and disease. Genome Medicine, 3(3), p.14.
- Nicholson, J., Holmes, E., Kinross, J., Burcelin, R., Gibson, G., Jia, W. and Pettersson, S. (2012). Host-Gut Microbiota Metabolic Interactions. Science, 336(6086), pp.1262-1267.
- Yatsunenko, T., Rey, F., Manary, M., Trehan, I., Dominguez-Bello, M., Contreras, M., Magris, M., Hidalgo, G., Baldassano, R., Anokhin, A., Heath, A., Warner, B., Reeder, J., Kuczynski, J., Caporaso, J., Lozupone, C., Lauber, C., Clemente, J., Knights, D., Knight, R. and Gordon, J. (2012). Human gut microbiome viewed across age and geography. Nature.
- Natividad, J. and Verdu, E. (2013). Modulation of intestinal barrier by intestinal microbiota: Pathological and therapeutic implications. Pharmacological Research, 69(1), pp.42-51.
- den Besten, G., van Eunen, K., Groen, A., Venema, K., Reijngoud, D. and Bakker, B. (2013). The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. Journal of Lipid Research, 54(9), pp.2325-2340.
- Dominguez-Bello, M., Costello, E., Contreras, M., Magris, M., Hidalgo, G., Fierer, N. and Knight, R. (2010). Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proceedings of the National Academy of Sciences, 107(26), pp.11971-11975.
- Neu, J. and Rushing, J. (2011). Cesarean Versus Vaginal Delivery: Long-term Infant Outcomes and the Hygiene Hypothesis. Clinics in Perinatology, 38(2), pp.321-331.
- O’Sullivan, A., Farver, M. and Smilowitz, J. (2015). The Influence of Early Infant-Feeding Practices on the Intestinal Microbiome and Body Composition in Infants. Nutrition and Metabolic Insights, 8s1, p.NMI.S41125.
- Heiman, M. and Greenway, F. (2016). A healthy gastrointestinal microbiome is dependent on dietary diversity. Molecular Metabolism, 5(5), pp.317-320.
- De Filippo, C., Cavalieri, D., Di Paola, M., Ramazzotti, M., Poullet, J., Massart, S., Collini, S., Pieraccini, G. and Lionetti, P. (2010). Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proceedings of the National Academy of Sciences, 107(33), pp.14691-14696.
- Ou, J., Carbonero, F., Zoetendal, E., DeLany, J., Wang, M., Newton, K., Gaskins, H. and O’Keefe, S. (2013). Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. American Journal of Clinical Nutrition, 98(1), pp.111-120.
- Graf, D., Di Cagno, R., Fåk, F., Flint, H., Nyman, M., Saarela, M. and Watzl, B. (2015). Contribution of diet to the composition of the human gut microbiota. Microbial Ecology in Health & Disease, 26(0).
- Walker, A., Ince, J., Duncan, S., Webster, L., Holtrop, G., Ze, X., Brown, D., Stares, M., Scott, P., Bergerat, A., Louis, P., McIntosh, F., Johnstone, A., Lobley, G., Parkhill, J. and Flint, H. (2010). Dominant and diet-responsive groups of bacteria within the human colonic microbiota. The ISME Journal, 5(2), pp.220-230.
- David, L., Maurice, C., Carmody, R., Gootenberg, D., Button, J., Wolfe, B., Ling, A., Devlin, A., Varma, Y., Fischbach, M., Biddinger, S., Dutton, R. and Turnbaugh, P. (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature, 505(7484), pp.559-563.
- Simpson, H. and Campbell, B. (2015). Review article: dietary fibre-microbiota interactions. Alimentary Pharmacology & Therapeutics, 42(2), pp.158-179.
- Singh, R., Chang, H., Yan, D., Lee, K., Ucmak, D., Wong, K., Abrouk, M., Farahnik, B., Nakamura, M., Zhu, T., Bhutani, T. and Liao, W. (2017). Influence of diet on the gut microbiome and implications for human health. Journal of Translational Medicine, 15(1).
- Devkota, S. and Chang, E. (2015). Interactions between Diet, Bile Acid Metabolism, Gut Microbiota, and Inflammatory Bowel Diseases. Digestive Diseases, 33(3), pp.351-356.
- Russell, W., Gratz, S., Duncan, S., Holtrop, G., Ince, J., Scobbie, L., Duncan, G., Johnstone, A., Lobley, G., Wallace, R., Duthie, G. and Flint, H. (2011). High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. American Journal of Clinical Nutrition, 93(5), pp.1062-1072.
- Brinkworth, G., Noakes, M., Clifton, P. and Bird, A. (2009). Comparative effects of very low-carbohydrate, high-fat and high-carbohydrate, low-fat weight-loss diets on bowel habit and faecal short-chain fatty acids and bacterial populations. British Journal of Nutrition, 101(10), p.1493.
- Peterson, D., Frank, D., Pace, N. and Gordon, J. (2008). Metagenomic Approaches for Defining the Pathogenesis of Inflammatory Bowel Diseases. Cell Host & Microbe, 3(6), pp.417-427.
- Martin, H., Campbell, B., Hart, C., Mpofu, C., Nayar, M., Singh, R., Englyst, H., Williams, H. and Rhodes, J. (2004). Enhanced Escherichia coli adherence and invasion in Crohn’s disease and colon cancer. Gastroenterology, 127(1), pp.80-93.
- Chassaing, B., Koren, O., Goodrich, J., Poole, A., Srinivasan, S., Ley, R. and Gewirtz, A. (2015). Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature, 519(7541), pp.92-96.
- O’Keefe, S., Li, J., Lahti, L., Ou, J., Carbonero, F., Mohammed, K., Posma, J., Kinross, J., Wahl, E., Ruder, E., Vipperla, K., Naidoo, V., Mtshali, L., Tims, S., Puylaert, P., DeLany, J., Krasinskas, A., Benefiel, A., Kaseb, H., Newton, K., Nicholson, J., de Vos, W., Gaskins, H. and Zoetendal, E. (2015). Fat, fibre and cancer risk in African Americans and rural Africans. Nature Communications, 6, p.6342.
- Ley, R., Turnbaugh, P., Klein, S. and Gordon, J. (2006). Microbial ecology: Human gut microbes associated with obesity. Nature, 444(7122), pp.1022-1023.
- Turnbaugh, P., Ley, R., Mahowald, M., Magrini, V., Mardis, E. and Gordon, J. (2006). An obesity-associated gut microbiome with increased capacity for energy harvest. Nature, 444(7122), pp.1027-131.
- Ojeda, P., Bobe, A., Dolan, K., Leone, V. and Martinez, K. (2016). Nutritional modulation of gut microbiota — the impact on metabolic disease pathophysiology. The Journal of Nutritional Biochemistry, 28, pp.191-200.
- Vrieze, A., Van Nood, E., Holleman, F., Salojärvi, J., Kootte, R., Bartelsman, J., Dallinga–Thie, G., Ackermans, M., Serlie, M., Oozeer, R., Derrien, M., Druesne, A., Van Hylckama Vlieg, J., Bloks, V., Groen, A., Heilig, H., Zoetendal, E., Stroes, E., de Vos, W., Hoekstra, J. and Nieuwdorp, M. (2012). Transfer of Intestinal Microbiota From Lean Donors Increases Insulin Sensitivity in Individuals With Metabolic Syndrome. Gastroenterology, 143(4), pp.913-916.e7.
- Swinburn, B., Sacks, G. and Ravussin, E. (2009). Increased food energy supply is more than sufficient to explain the US epidemic of obesity. The American Journal of Clinical Nutrition, 90(6), pp.1453-1456.
- Haro, C., Garcia-Carpintero, S., Alcala-Diaz, J., Gomez-Delgado, F., Delgado-Lista, J., Perez-Martinez, P., Rangel Zuñiga, O., Quintana-Navarro, G., Landa, B., Clemente, J., Lopez-Miranda, J., Camargo, A. and Perez-Jimenez, F. (2016). The gut microbial community in metabolic syndrome patients is modified by diet. The Journal of Nutritional Biochemistry, 27, pp.27-31.
- Dinan, T., Stilling, R., Stanton, C. and Cryan, J. (2015). Collective unconscious: How gut microbes shape human behavior. Journal of Psychiatric Research, 63, pp.1-9.
- Clarke, G., McKernan, D., Gaszner, G., Quigley, E., Cryan, J. and Dinan, T. (2012). A Distinct Profile of Tryptophan Metabolism along the Kynurenine Pathway Downstream of Toll-Like Receptor Activation in Irritable Bowel Syndrome. Frontiers in Pharmacology, 3.
- Russell, W., Hoyles, L., Flint, H. and Dumas, M. (2013). Colonic bacterial metabolites and human health. Current Opinion in Microbiology, 16(3), pp.246-254.
- Tillisch, K., Labus, J., Kilpatrick, L., Jiang, Z., Stains, J., Ebrat, B., Guyonnet, D., Legrain–Raspaud, S., Trotin, B., Naliboff, B. and Mayer, E. (2013). Consumption of Fermented Milk Product With Probiotic Modulates Brain Activity. Gastroenterology, 144(7), pp.1394-1401.e4.
- Messaoudi, M., Lalonde, R., Violle, N., Javelot, H., Desor, D., Nejdi, A., Bisson, J., Rougeot, C., Pichelin, M., Cazaubiel, M. and Cazaubiel, J. (2010). Assessment of psychotropic-like properties of a probiotic formulation ( Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. British Journal of Nutrition, 105(05), pp.755-764.
- Rea, K., Dinan, T. and Cryan, J. (2016). The microbiome: A key regulator of stress and neuroinflammation. Neurobiology of Stress, 4, pp.23-33.
- Neufeld, K. and Foster, J. (2009). Effects of gut microbiota on the brain: implications for psychiatry. J Psychiatry Neurosci., May 34(3), pp.230–231.
- Parracho, H. (2005). Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. Journal of Medical Microbiology, 54(10), pp.987-991.
- Williams, B., Hornig, M., Buie, T., Bauman, M., Cho Paik, M., Wick, I., Bennett, A., Jabado, O., Hirschberg, D. and Lipkin, W. (2011). Impaired Carbohydrate Digestion and Transport and Mucosal Dysbiosis in the Intestines of Children with Autism and Gastrointestinal Disturbances. PLoS ONE, 6(9), p.e24585.
- Santocchi, E., Guiducci, L., Fulceri, F., Billeci, L., Buzzigoli, E., Apicella, F., Calderoni, S., Grossi, E., Morales, M. and Muratori, F. (2016). Gut to brain interaction in Autism Spectrum Disorders: a randomized controlled trial on the role of probiotics on clinical, biochemical and neurophysiological parameters. BMC Psychiatry, 16(1).
- Gibson, G., Probert, H., Loo, J., Rastall, R. and Roberfroid, M. (2004). Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutrition Research Reviews, 17(02), p.259.
- Van Loo, J., Coussement, P., De Leenheer, L., Hoebregs, H. and Smits, G. (1995). On the presence of Inulin and Oligofructose as natural ingredients in the western diet. Critical Reviews in Food Science and Nutrition, 35(6), pp.525-552.
- Brownawell, A., Caers, W., Gibson, G., Kendall, C., Lewis, K., Ringel, Y. and Slavin, J. (2012). Prebiotics and the Health Benefits of Fiber: Current Regulatory Status, Future Research, and Goals. Journal of Nutrition, 142(5), pp.962-974.
- Bouhnik, Y., Raskine, L., Simoneau, G., Vicaut, E., Neut, C., Flourié, B., Brouns, F. and Bornet, F. (2004). The capacity of nondigestible carbohydrates to stimulate fecal bifidobacteria in healthy humans: a double-blind, randomized, placebo-controlled, parallel-group, dose-response relation study. Am J Clin Nutr, (80), pp.1658–64.
- Patel, R. and DuPont, H. (2015). New Approaches for Bacteriotherapy: Prebiotics, New-Generation Probiotics, and Synbiotics. Clinical Infectious Diseases, 60(suppl 2), pp.S108-S121.
- Roberts, C., Keita, A., Duncan, S., O’Kennedy, N., Soderholm, J., Rhodes, J. and Campbell, B. (2010). Translocation of Crohn’s disease Escherichia coli across M-cells: contrasting effects of soluble plant fibres and emulsifiers. Gut, 59(10), pp.1331-1339.
- Turner, D., Shah, P., Steinhart, A., Zlotkin, S. and Griffiths, A. (2011). Maintenance of remission in inflammatory bowel disease using omega-3 fatty acids (fish oil): A systematic review and meta-analyses. Inflammatory Bowel Diseases, 17(1), pp.336-345.
- Uchiyama, K., Nakamura, M., Odahara, S., Koido, S., Katahira, K., Shiraishi, H., Ohkusa, T., Fujise, K. and Tajiri, H. (2010). N-3 polyunsaturated fatty acid diet therapy for patients with inflammatory bowel disease. Inflammatory Bowel Diseases, 16(10), pp.1696-1707.
- Clark, A. and Mach, N. (2016). Role of Vitamin D in the Hygiene Hypothesis: The Interplay between Vitamin D, Vitamin D Receptors, Gut Microbiota, and Immune Response. Frontiers in Immunology, 7.
- Flanagan, P., Chiewchengchol, D., Wright, H., Edwards, S., Alswied, A., Satsangi, J., Subramanian, S., Rhodes, J. and Campbell, B. (2015). Killing of Escherichia coli by Crohnʼs Disease Monocyte-derived Macrophages and Its Enhancement by Hydroxychloroquine and Vitamin D. Inflammatory Bowel Diseases, 21(7), pp.1499-1510.
Clarke, G., Grenham, S., Scully, P., Fitzgerald, P., Moloney, R., Shanahan, F., Dinan, T. and Cryan, J. (2012). The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Molecular Psychiatry, 18(6), pp.666-673.