The word circadian is derived from the Latin circa, meaning around, and dies, meaning day: around the day. All organisms on the planet have an organisation of physiological processes based on the two distinct environmental contrasts that occur as a result of Earth’s 24-hour daily rotation: night and day, dark and light. These contrasts are accompanied with other environmental changes relevant to any species, including food availability, temperature, and susceptibility to predation.
The ability to anticipate changes in the environment confers a distinct evolutionary advantage on any species. Physiological processes and behaviours follow circadian rhythms corresponding to the 24-hour cycle of light and darkness, and are entrained by various “zeitgebers” [the term in the research field, German for “time giver”] of time of day. In humans, the light period is also the waking phase, active phase, and phase of food foraging and consumption. The dark period is the sleeping phase, inactive phase, and fasting phase.
The master controller of the circadian “clock” is located in a region of the hypothalamus known as the suprachiasmatic nucleus [SCN]. The primary environmental factor driving circadian entrainment in the SCN is light: a specialised set of retinal cells communicate information about light to the SCN indicating whether it is day or night. Picture a blue sky on a clear day – that colour is not random to human physiology, it is a specific wavelength of shortwave blue light of 460-480 nanometers which provides the strongest signal to the SCN that it is the day/waking/active phase, [1]. Based on what input the SCN receives, it will synchronise the appropriate physiological requirements of that period to the light cues –,to wake, to eat, to digest, energy availability and storage [2].
However, peripheral tissues – including the liver, kidneys, pancreas, muscle, and adipose tissues – are also entrained by timing of food intake. The circadian system relies on synchrony between the central and peripheral clocks for optimal physiological function [3]. Thus, the inputs are vital to the integrity of our circadian system, and sending different or unusual inputs – inconsistent light signals, irregular meal patterns, curtailed sleep – can alter the outputs, including hormonal secretions, substrate oxidation and storage, hunger and appetite [3].
It is well established in the literature that circadian desynchronization, for example from shift work, is a primary risk factor for cardiometabolic disease [4]. This is because when food intake is desynchronized from the normally dichotomous circadian patterns of day/light/feed/active and night/dark/fast/inactive, control of metabolic processes become decoupled from the SCN, which is primarily entrained to light signals as opposed to food intake [3]. Traditionally, the focus of research into the importance of the circadian system for metabolic health focused on extreme situations like jet-lag and shift work. However, the effects of less extreme perturbations on metabolic health are coming to light.
Associations Between Circadian Disruption and Metabolic Health
As light is the primary time cue for the central clock, exposure to light has implications for metabolic health, potentially both positive and negative depending on timing of exposure. People in industrialised societies spend up to 88% of time in enclosed buildings, resulting in 4-fold less natural light exposure during the day [5][6]. To give you some perspective on this, light is measured in ‘lux’, and natural daytime light can be anywhere between 2,000 to 10,000lux while average indoor lighting can be less than 500lux, which lacks the minimum level of intensity for circadian entrainment [7]. Recent associative studies have found that greater morning light exposure correlates to lower body fat levels and better appetite regulation [1][20].
Conversely, at nighttime people in industrialized societies are exposed to artificial light, in particular from electronic devices which emit shortwave blue light, the very same light intensity emitted during the day. Melatonin, the primary hormone produced at night in response to darkness, is maximally suppressed by shortwave blue light [1]. This may be problematic as melatonin receptors have been identified in the pancreas and modulate insulin secretion [8]. In the Nurses’ Health Study, women with the lowest melatonin levels at baseline were more likely to have developed T2DM when followed up with 12 years later [9]. The effects of this alteration in our natural light environments and normally dichotomous periods of wake/sleep coinciding with feed/fast are both indirect and direct.
Indirect effects include ‘social jetlag’, and the effect of extended evening illumination on meal timing. ‘Social jetlag’ is the discrepancy between actual sleep time during the week, with enforced early waking or late evenings, and how much sleep an individual would actually need if they were to sleep freely. The issue is that many people keep social timing that is significantly different to circadian timing, which dysregulates circadian rhythms. Several recent large cohort studies have found that greater degrees of social jetlag are significantly, inversely associated with rates of metabolic disease, in particular type-2 diabetes and obesity, associations which remain after controlling for variables like sleep duration and “chronotype” [whether someone is a morning type or evening type] [10][11].
In addition, both the duration and intensity of artificial light exposure at night are associated with metabolic dysfunction, obesity and abnormal lipid profiles [12][13]. One issue in relation to this observation is the indirect effect of extended evening wakefulness, which increases propensity for late-night eating, and late meal timing is associated with higher total daily calorie intake and increased BMI, independent of sleep timing and duration [14][15]. Nutrient utilisation and energy expenditure are regulated by the circadian system, and late-night eating alters the dichotomous feed/fast period and causes circadian rhythms in the digestive system, pancreas, liver, and other metabolic peripheral tissues to become offset from the central clock [16].
Underlying Mechanisms and Intervention Studies
What could be giving rise to these observations? An underappreciated feature of melatonin is its interaction with insulin. Feeding in humans occurs – or should occur – during the light/waking phase, when melatonin levels are low. When melatonin is elevated during the biological night, insulin sensitivity is impaired and insulin secretion increases [17]. In an intervention study designed to replicate the effects of early morning waking, elevated melatonin impaired glucose tolerance in response to a meal provided 1-hour after waking in subjects sleeping 5-hours vs. subjects sleeping 9-hours [17]. The underlying insulin resistance and exaggerated pancreatic beta-cell insulin response to nutrient ingestion during the biological night may explain why the increased cardiometabolic risk of shift work patterns is observed independent of total calorie intake [18].
Another underappreciated element of nutritional pharmacology is the crucial role the post-prandial period plays in metabolic health. Cardiovascular disease has long been described as a “post-prandial phenomenon” – a phrase which reflects that the mechanisms of atherosclerosis are profoundly influenced by how well someone processes dietary fat and circulating fatty acids in this period. Circulating non-esterified fatty acids exhibit circadian variance with their peak in the evening, an increase which occurs independent of food intake [8]. Increases in NEFA are strongly implicated in hepatic insulin resistance, hyperglycemia, hypertriglyceridemia: a clustering of cardiometabolic risk factors [19]. This is compounded by post-prandial lipemia from late-night eating.
The limited body of human intervention studies to date indicate that this synchrony between the central circadian clock and peripheral tissues, regulated by light and meal timing, respectively, confers added advantages for metabolic health. Danilenko et al. [20] investigated the effects of bright morning light therapy on a weight loss program in overweight women; the crossover design used a deactivated ion light as a light placebo. Over 3-weeks of the intervention, light therapy resulted in a significant reduction in fat mass and percent body fat, and reduction in appetite [20]. In another trial exposing subjects to 6-hours blue light exposure per day divided into two 3-hour sessions, markers of insulin resistance increased suggesting that chronic exposure to shortwave blue light may impact glucose regulation and alter metabolic function [21]. This is consistent with animal model data in which continual exposure to artificial light increases markers of insulin resistance [22]
This reduction in appetite from light exposure has been repeated elsewhere: in a trial designed to replicate sleep curtailment, a known variable which increases hunger and appetite from increased ghrelin and reduced leptin levels, blue light exposure in the morning mitigated these hormonal responses and led to reductions in ghrelin signalling and increased circulating leptin [1]. The primary mechanism underlying these effects is the physiological arousal which blue light promotes [1][20]. This may result in increased diet-induced thermogenesis, which itself has circadian variance peaking in the early part of the day [23].
While this is a nascent research field, taken as a whole these studies show us that:
- The physiological responses to light cues appear to be quite pronounced in humans;
- Morning blue light therapy may be a novel intervention to assist with hunger/appetite regulation and promote physiological arousal resulting in greater reductions in fat mass;
- Extended evening illumination is an environmental risk factor for obesity by extending opportunities to eat and causing desynchronized circadian regulation of metabolism.
Circadian Regulation of Energy Balance
Another underappreciated aspect of circadian architecture is that the circadian system and the central control of energy homeostasis overlap in the hypothalamus. In particular, both ghrelin and leptin receptors are present in the SCN [24]. Ghrelin, the gut-derived hormone which stimulates hunger, is entrained in response to fixed feeding patterns and provides signaling between the peripheral clocks [entrained by feeding] and central clocks [entrained by light] [3]. Food reward and motivation systems are synchronised to the circadian clock, allowing metabolic processes to anticipate nutrient intake and metabolism [25]. As a result, circadian misalignment dysregulates appetite and central control of energy balance [26].
Due to the entrainment provided to peripheral tissues by nutrient ingestion, regular meal timing is essential to circadian alignment. An interesting trial in humans found that irregular meal patterns decreases diet-induced thermogenesis compared an isocaloric diet fed with regular meal timing [27]. This is not to suggest increase meal frequency is the variable, it is demonstrating that erratic meal patterns are disruptive to circadian control of metabolism [28]. This may be particularly important to carbohydrate metabolism, as circadian disruption disturbs glucose metabolism and negatively impacts carbohydrate oxidation [3]. Conversely, sustained regular meal timing shifts substrate utilisation to favour fat oxidation over carbohydrate oxidation and lipid storage [3].
This regulatory role of substrate digestion and metabolism is central to the role of circadian dysregulation in metabolic health. Rhythms in energy intake must correspond with rhythms in the mechanisms of nutrient digestion and absorption in order to optimize metabolic function [29]. The gastrointestinal tract displays rhythms at multiple levels, including motility, enzyme secretion and activity, and macronutrient absorption [8][25]. Beyond those initial processes of nutrient digestion and absorption, the rhythmicity of different peripheral tissues differs based on time of day: adipose tissue sensitivity to insulin peaks during the middle of the day, and promotes fat accumulation in the evening [30]. Pancreatic clocks ensure insulin action is heightened during the waking/feeding phase, but the timing of glucose rhythms can be shifted in the liver by late meal timing [31].
While there are gaps in the literature, what is clear is that hunger, appetite, nutrient metabolism and substrate oxidation are all strongly influenced by circadian regulation, and the evidence to date supports the proposition that dysynchrony between peripheral clocks and the central circadian clock undermines control of energy balance in humans.
Gaps in Literature: Time-Restricted Feeding & Circadian Arguments in Nutrient Timing
Different methodologies have evolved by reference to circadian research in relation to timing of food intake, with many popular strategies shifting a majority of calories to the evening, and/or accompanied by a morning fast. My difficulty, as someone researching in the field, is when strong arguments are made in favour of an optimal human feeding timing pattern when the body of literature is inconsistent. Two trials in particular illustrate this point.
The first was an RCT in overweight/obese women feeding isocaloric 1,400kcal/d diets, but switching calorie intake between breakfast and dinner meals (23). Lunch was controlled for 500kcal, one group consumed 700kcal at breakfast and 200kcal at dinner compared to a group consuming the opposite. Importantly, protein intake was >30g in each meal, a threshold associated with improved satiety [32]. Over 12-weeks, the 700kcal breakfast group lost 8.7kg vs. 3.6kg in the 700kcal dinner group, and improved fasting glucose and insulin sensitivity indices [23]. In particular, ghrelin was higher in the dinner group which corresponded to high subjective hunger and lower satiety levels (Ibid.), an interesting observation given that ghrelin peaks in the evening around 7pm [33]. Conversely, another RCT in overweight/obese adults found greater weight loss during a calorie-restricted weight loss diet where subjects consumed a majority of their carbohydrate intake in the evening meal [34]. Calorie intake in this study was similar [13,00-1,500kcal/d] as the prior study, however, no details were provided in relation to the meal-by-meal calorie breakdown, making a comparison with the prior study difficult. One of the interesting findings in this study was that this feeding pattern attenuated the decrease in leptin which accompanies energy restriction, while preserving adiponectin levels, an insulin sensitising hormone [34]. However, the difference in weight loss in this study was much less significant than in the prior study over the same 12-week timeframe, while other anthropometric measurements were not significantly different between control diet with carbohydrate spaced throughout the day and intervention diet [34][23].
There is certainly an argument to be made that high refined carbohydrate intake at breakfast stimulates hunger and appetite, an effect which may be mediated by the augmented insulin response from the circadian increase in morning cortisol, promoting rapid carbohydrate metabolism [35]. However, this experimental human study used a dose of hydrocortisone which may not quite reflect the interaction between diurnal fluctuations in cortisol, and feeding-induced insulin secretion. Nonetheless, food-based intervention studies have found that reducing the carbohydrate content of breakfast from 55% to 43% energy and replacing with fat (protein was controlled for) reduced hunger and appetite 4-hours later [36]. It is arguable that this isn’t exclusively a circadian influence, however, our behavioural patterns inevitably overlap with our circadian rhythms, and consideration of macronutrient balance in the morning may improve appetite/hunger, and enhance dietary adherence.
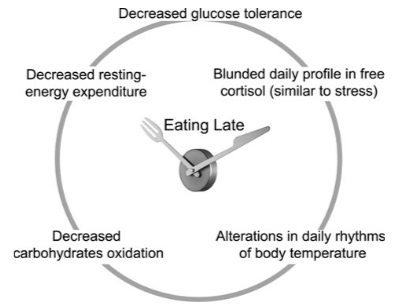
Overall, from the perspective of circadian timing of food intake, the body of human research does suggest that earlier eating of main meals (i.e. a particular distribution of calories) is more beneficial. In a study in Spain, an area with traditional late-night eating patterns, overweight/obese subjects on a weight loss program who consumed their main meal before 3pm lost significantly more weight than those consuming their main meal after 3pm, despite similar total energy intake [diet wasn’t controlled for] and sleep duration [37]. An interesting observation in this study, consistent with the wider literature in the field, is that late eaters were also evening chronotypes, i.e. “night owls” [37]. Perhaps the best randomized controlled human study in this area analysed the effects of early [1pm] vs. late [4.30pm] lunch timing in a crossover design using lean healthy women where breakfast, lunch and dinner were standardised [38]. Measures of resting energy expenditure were taken using indirect calomitery, and glucose tolerance was assessed along with circadian measures including cortisol and temperature (38). Late timing of lunch (which as subjects consumed dinner meant a majority calories overall consumed later in the day) resulted in metabolic alterations in lean women typically characteristic of overweight individuals: decreased resting energy expenditure, reduced carbohydrate oxidation, decreased glucose tolerance, and blunted cortisol profiles [38].
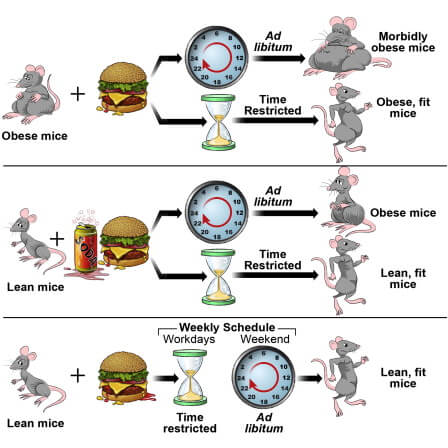
Another fascinating element of chrononutrition research is that time-restricted feeding [TRF] to the waking/active phase prevents rodents from obesity, without altering nutrient composition or calorie intake [39][40]. In one study, mice fed an obesity-inducing high fat diet were protected against obesity by time-restricting their food access to an 8-hour timeframe during their biological day, compared to mice consuming the same diet but with ad libitum access across their day [39]. Mice in the TRF group increased daily activity, significantly increased diet-induced thermogenesis, and improved circadian rhythms in substrate utilization, collectively leading to reduced adiposity and preservation of glucose tolerance [39].
Another fascinating study developed on the examined whether the protective effect extended to pre-existing obesity, different obesity-inducing diets, and different eating patterns [40]. Mice induced with obesity reduced body weight and maintained that weight loss after switching from ad libitum feeding to a TRF regimen [40]. In response to feeding a high fat + high sucrose diet, the ad lib group increased adiposity by 42% while the TRF increased by 21%, despite the same calorie intake [40]. There were also linear dose-responses between duration of food access and degree of weight gain: in mice allowed access to food for 9, 12, or 15-hours, longer access led to increased adiposity despite equal food consumption [40].
In humans, there is some emerging, limited evidence of benefits to TRF: in one study, regularising erratic eating patterns and shortening the habitual feeding period from 14hrs to 10-11hrs decreased energy intake, body mass, and increased sleep quality [41]. Perhaps more relevant to Biolayne.com readers, a controlled trial in resistance trained males with meal timing either at 8am, 1pm and 8pm vs. an 8-hour TRF pattern with meals at 1pm, 4pm, and 8pm with macronutrients matched found significantly greater fat mass loss [1.6kg vs. 0.3kg] in the TRF group [42]. The TRF patterns significantly elevated adiponectin, and improved other metabolic biomarkers including glucose, insulin, and triglycerides (Ibid). However, dietary intake was based on 7-day recall, and differences in energy intake between groups could not be ruled out [42]. Overall, the emerging human models lend some support – albeit much less dramatic in effect – to benefits of TRF patterns.
One point that must be made clear: TRF is not to be considered just another intermittent fasting style. They are distinct, and many IF paradigms all but ignore time-of-day nutrient intake. From a circadian chronobiology perspective, time-restricted feeding provides a clear feeding/fasting cycle that is consistent with the waking/sleeping and light/dark phases, syncing peripheral circadian metabolic functions with our central, light-driven circadian clock [43].
Conclusions: Recommendations for Improving Circadian System Health
Based on the body of research to date, there are recommendations we can broadly make to improve metabolic health through improving the circadian regulation. Those are:
- Don’t skimp on sleep. Despite anything you’ve heard, 7-9-hours a night is needed.
- Create distinct light environments.
- Mornings: Aim to get 30mins outdoor natural light exposure. If you live in a region of the world with dark winter mornings, use a blue-light box or sunrise lamp for 15-30mins within an hour of waking [options here and here].
- Evenings: The key is minimizing light intensity and blue-light exposure. The lowest hanging fruit is as follows:
- Use soft side lighting and keep rooms lowly lit.
- Download the software F.lux and use it for your laptop, it will naturally dim blue light from your screen at sunset.
- Make your bedroom a tech-free zone, and keep it blacked out during sleep.
- Turn off intense blue-lighting – like TV’s – around an hour before bed. Give melatonin a chance to rise.
For extra credit, you can wear blue-light blocking glasses, which prevent melatonin suppression and improve sleep quality [44].
- Be consistent with regular meal timing: don’t have erratic eating patterns. Whether you like 3 meals or 5, meal frequency isn’t the factor: it’s being consistent with the daily timing of those meals that is important to entrain peripheral tissues and enhance nutrient metabolism.
- Nutrient timing. While this picture is incomplete, from a circadian perspective, what evidence there is suggests that consuming your largest daily calorie meal earlier in the day, i.e. before 3pm, has positive metabolic health benefits.
- Time-restrict feeding. This is not saying “do IF” – TRF can be even an 11-hour daily window. The goal is to regularise your meal timing and provide your body with distinct feed/fast cycles that align with your daily wake/sleep and active/inactive cycles.
While the research field from a nutrition perspective is still in its infancy, one thing that is abundantly clear is that when we eat is an important determinant of metabolic health. Where we need to fill in gaps is more specific data on how what we eat interacts with when we eat it to modify disease risk or enhance health. Nonetheless, this is promising for obesity prevention and treatment and we have enough data to make simple diet and lifestyle recommendations to improve metabolic health from a circadian perspective.
References
- Figueiro, M., Plitnick, B. and Rea, M. (2012). Light Modulates Leptin and Ghrelin in Sleep-Restricted Adults. International Journal of Endocrinology, 2012, pp.1-6.
- Lopez-Minguez, J., Gómez-Abellán, P. and Garaulet, M. (2016). Circadian rhythms, food timing and obesity. Proceedings of the Nutrition Society, 75(04), pp.501-511.
- Westerterp-Plantenga, M. (2016). Sleep, circadian rhythm and body weight: parallel developments. Proceedings of the Nutrition Society, 75(04), pp.431-439.
- Depner, C., Stothard, E. and Wright, K. (2014). Metabolic Consequences of Sleep and Circadian Disorders. Current Diabetes Reports, 14(7).
- Klepeis, N., Nelson, W., Ott, W., Robinson, J., Tsang, A., Switzer, P., Behar, J., Hern, S. and Engelmann, W. (2001). The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. Journal of Exposure Analysis and Environmental Epidemiology, 11(3), pp.231-252.
- Wright, K., McHill, A., Birks, B., Griffin, B., Rusterholz, T. and Chinoy, E. (2013). Entrainment of the Human Circadian Clock to the Natural Light-Dark Cycle. Current Biology, 23(16), pp.1554-1558.
- Bonmati-Carrion, M., Arguelles-Prieto, R., Martinez-Madrid, M., Reiter, R., Hardeland, R., Rol, M. and Madrid, J. (2014). Protecting the Melatonin Rhythm through Circadian Healthy Light Exposure. International Journal of Molecular Sciences, 15(12), pp.23448-23500.
- Bailey, S., Udoh, U. and Young, M. (2014). Circadian Regulation of Metabolism. J Endocrinol, 222(2), pp.75-96.
- McMullan, C., Schernhammer, E., Rimm, E., Hu, F. and Forman, J. (2013). Melatonin Secretion and the Incidence of Type 2 Diabetes. JAMA, 309(13), p.1388.
- Roenneberg, T., Allebrandt, K., Merrow, M. and Vetter, C. (2012). Social Jetlag and Obesity. Current Biology, 22(10), pp.939-943.
- Parsons, M., Moffitt, T., Gregory, A., Goldman-Mellor, S., Nolan, P., Poulton, R. and Caspi, A. (2014). Social jetlag, obesity and metabolic disorder: investigation in a cohort study. International Journal of Obesity, 39(5), pp.842-848.
- Obayashi, K., Saeki, K., Iwamoto, J., Okamoto, N., Tomioka, K., Nezu, S., Ikada, Y. and Kurumatani, N. (2013). Exposure to Light at Night, Nocturnal Urinary Melatonin Excretion, and Obesity/Dyslipidemia in the Elderly: A Cross-Sectional Analysis of the HEIJO-KYO Study. The Journal of Clinical Endocrinology & Metabolism, 98(1), pp.337-344.
- Reid, K., Santostasi, G., Baron, K., Wilson, J., Kang, J. and Zee, P. (2014). Timing and Intensity of Light Correlate with Body Weight in Adults. PLoS ONE, 9(4), p.e92251.
- Baron, K., Reid, K., Horn, L. and Zee, P. (2013). Contribution of evening macronutrient intake to total caloric intake and body mass index. Appetite, 60, pp.246-251.
- Reid, K., Baron, K. and Zee, P. (2014). Meal timing influences daily caloric intake in healthy adults. Nutrition Research, 34(11), pp.930-935.
- Oike, H., Oishi, K. and Kobori, M. (2014). Nutrients, Clock Genes, and Chrononutrition. Current Nutrition Reports, 3(3), pp.204-212.
- Eckel, R., Depner, C., Perreault, L., Markwald, R., Smith, M., McHill, A., Higgins, J., Melanson, E. and Wright, K. (2015). Morning Circadian Misalignment during Short Sleep Duration Impacts Insulin Sensitivity. Current Biology, 25(22), pp.3004-3010.
- Bonham, M., Bonnell, E. and Huggins, C. (2016). Energy intake of shift workers compared to fixed day workers: A systematic review and meta-analysis. Chronobiology International, 33(8), pp.1086-1100.
- Kotronen, A. and Yki-Jarvinen, H. (2007). Fatty Liver: A Novel Component of the Metabolic Syndrome. Arteriosclerosis, Thrombosis, and Vascular Biology, 28(1), pp.27-38.
- Danilenko, K., Mustafina, S. and Pechenkina, E. (2013). Bright Light for Weight Loss: Results of a Controlled Crossover Trial. Obesity Facts, 6(1), pp.28-38.
- Cheung, I., Zee, P., Shalman, D., Malkani, R., Kang, J. and Reid, K. (2016). Morning and Evening Blue-Enriched Light Exposure Alters Metabolic Function in Normal Weight Adults. PLOS ONE, 11(5), p.e0155601.
- Fonken, L., Workman, J., Walton, J., Weil, Z., Morris, J., Haim, A. and Nelson, R. (2010). Light at night increases body mass by shifting the time of food intake. Proceedings of the National Academy of Sciences, 107(43), pp.18664-18669.
- Jakubowicz, D., Barnea, M., Wainstein, J. and Froy, O. (2013). High Caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity, 21(12), pp.2504-2512.
- Froy, O. (2010). Metabolism and Circadian Rhythms—Implications for Obesity. Endocrine Reviews, 31(1), pp.1-24.
- Young, M. and McGinnis, G. (2016). Circadian regulation of metabolic homeostasis: causes and consequences. Nature and Science of Sleep, p.163.
- Scheer, F., Hilton, M., Mantzoros, C. and Shea, S. (2009). Adverse metabolic and cardiovascular consequences of circadian misalignment. Proceedings of the National Academy of Sciences, 106(11), pp.4453-4458.
- Farshchi, H., Taylor, M. and Macdonald, I. (2004). Decreased thermic effect of food after an irregular compared with a regular meal pattern in healthy lean women. International Journal of Obesity, 28(5), pp.653-660.
- Mattson, M., Allison, D., Fontana, L., Harvie, M., Longo, V., Malaisse, W., Mosley, M., Notterpek, L., Ravussin, E., Scheer, F., Seyfried, T., Varady, K. and Panda, S. (2014). Meal frequency and timing in health and disease. Proceedings of the National Academy of Sciences, 111(47), pp.16647-16653.
- Eckel-Mahan, K. and Sassone-Corsi, P. (2013). Metabolism and the Circadian Clock Converge. Physiological Reviews, 93(1), pp.107-135.
- Bass, J. (2012). Circadian topology of metabolism. Nature, 491(7424), pp.348-356.
- Wehrens, S., Christou, S., Isherwood, C., Middleton, B., Gibbs, M., Archer, S., Skene, D. and Johnston, J. (2017). Meal Timing Regulates the Human Circadian System. Current Biology, 27(12), pp.1768-1775.e3.
- Phillips, S., Chevalier, S. and Leidy, H. (2016). Protein “requirements” beyond the RDA: implications for optimizing health. Applied Physiology, Nutrition, and Metabolism, 41(5), pp.565-572.
- Scheer, F., Morris, C. and Shea, S. (2013). The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity, 21(3), pp.421-423.
- Sofer, S., Eliraz, A., Kaplan, S., Voet, H., Fink, G., Kima, T. and Madar, Z. (2011). Greater Weight Loss and Hormonal Changes After 6 Months Diet With Carbohydrates Eaten Mostly at Dinner. Obesity, 19(10), pp.2006-2014.
- Vila, G., Krebs, M., Riedl, M., Baumgartner-Parzer, S., Clodi, M., Maier, C., Pacini, G. and Luger, A. (2010). Acute effects of hydrocortisone on the metabolic response to a glucose load: increase in the first-phase insulin secretion. European Journal of Endocrinology, 163(2), pp.225-231.
- Chandler-Laney, P., Morrison, S., Goree, L., Ellis, A., Casazza, K., Desmond, R. and Gower, B. (2014). Return of hunger following a relatively high carbohydrate breakfast is associated with earlier recorded glucose peak and nadir. Appetite, 80, pp.236-241.
- Garaulet, M., Gómez-Abellán, P., Alburquerque-Béjar, J., Lee, Y., Ordovás, J. and Scheer, F. (2013). Timing of food intake predicts weight loss effectiveness. International Journal of Obesity, 37(4), pp.624-624.
- Bandín, C., Scheer, F., Luque, A., Ávila-Gandía, V., Zamora, S., Madrid, J., Gómez-Abellán, P. and Garaulet, M. (2014). Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: A randomized, crossover trial. International Journal of Obesity, 39(5), pp.828-833.
- Hatori, M., Vollmers, C., Zarrinpar, A., DiTacchio, L., Bushong, E., Gill, S., Leblanc, M., Chaix, A., Joens, M., Fitzpatrick, J., Ellisman, M. and Panda, S. (2012). Time-Restricted Feeding without Reducing Caloric Intake Prevents Metabolic Diseases in Mice Fed a High-Fat Diet. Cell Metabolism, 15(6), pp.848-860.
- Chaix, A., Zarrinpar, A., Miu, P. and Panda, S. (2014). Time-Restricted Feeding Is a Preventative and Therapeutic Intervention against Diverse Nutritional Challenges. Cell Metabolism, 20(6), pp.991-1005.
- Gill, S. and Panda, S. (2015). A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metabolism, 22(5), pp.789-798.
- Moro, T., Tinsley, G., Bianco, A., Marcolin, G., Pacelli, Q., Battaglia, G., Palma, A., Gentil, P., Neri, M. and Paoli, A. (2016). Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. Journal of Translational Medicine, 14(1).
- Potter, G., Skene, D., Arendt, J., Cade, J., Grant, P. and Hardie, L. (2016). Circadian Rhythm and Sleep Disruption: Causes, Metabolic Consequences, and Countermeasures. Endocrine Reviews, 37(6), pp.584-608.
- van der Lely, S., Frey, S., Garbazza, C., Wirz-Justice, A., Jenni, O., Steiner, R., Wolf, S., Cajochen, C., Bromundt, V. and Schmidt, C. (2015). Blue Blocker Glasses as a Countermeasure for Alerting Effects of Evening Light-Emitting Diode Screen Exposure in Male Teenagers. Journal of Adolescent Health, 56(1), pp.113-119.




